Aniflazym
alt informationenConfidential Document
Takeda Property
Serratiopeptidase-10075 Ver.1.0
MANUFACTURE:
DESCRIPTION OF MANUFACTURING PROCESS AND PROCESS CONTROLS
Sato Yakuhin Kogyo Co., Ltd.
Danzen Tablets
3.2.P.3.3
Kazumichi Yamamoto Director
Pharmaceutical Technology Department Pharmaceutical Production Division Takeda Pharmaceutical Company Limited
June 2013
3.0. DESCRIPTION OF MANUFACTURING PROCESS AND PROCESS
4.0. REWORKING AND REPROCESSING...................................................................8
LIST OF FIGURES
Figure 1 Manufacturing Process Flow Diagram for Danzen Tablets...............................5
1.0. INTRODUCTION
This section provides information for the manufacture of Danzen tablets at Sato Yakuhin Kogyo Co., Ltd .
2.0. FLOW DIAGRAM
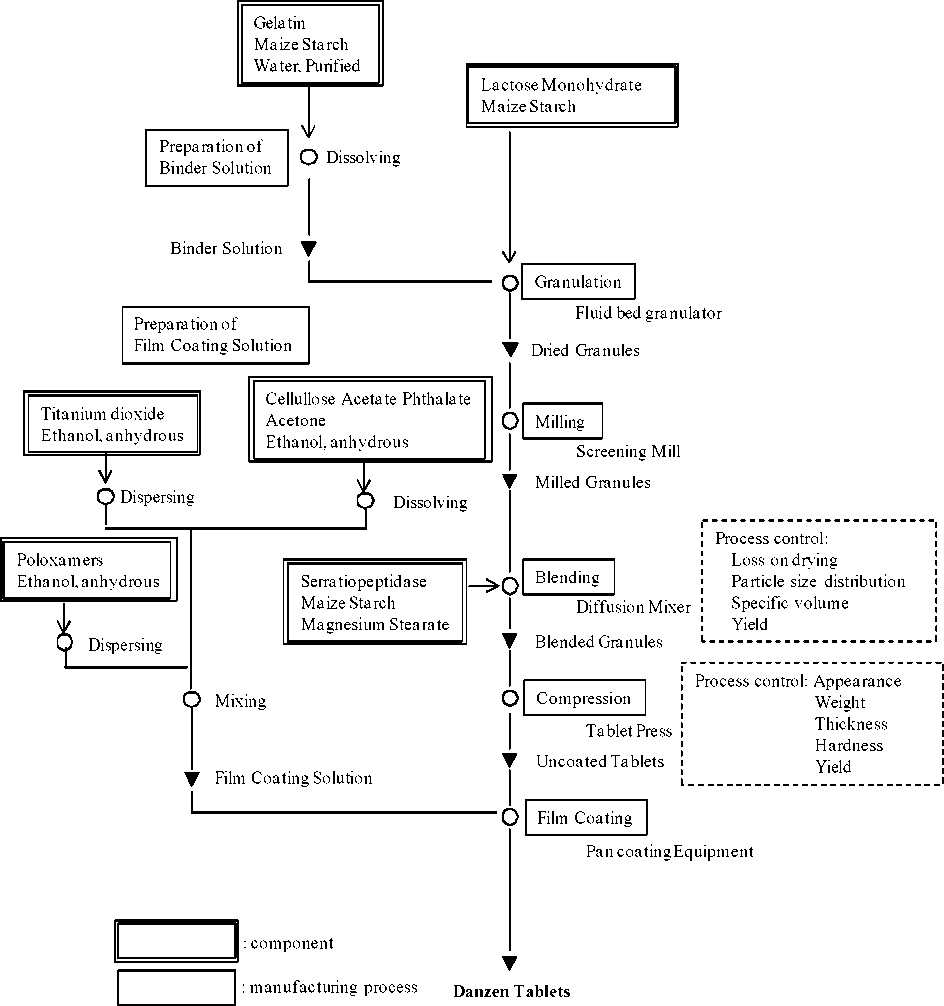
A flow diagram illustrating the manufacturing process for Danzen tablets is provided in Figure 1. Process controls performed at key process are indicated in boxes with dashed lines located at the right side of the diagram.
Manufacturing Process Flow Diagram for Danzen Tablets
3.0. DESCRIPTION OF MANUFACTURING PROCESS AND PROCESS CONTROLS
The manufacturing process for a typical batch size of approximately 880,000 for Danzen tablets is described in the narrative below.
Step Process Description
Granulation and milling
1 Gelatin and maize starch are dissolved in water, purified by stirring to prepare the binder solution.
2 Lactose monohydrate and maize starch are mixed and granulated by spraying the binder solution in a fluid bed granulator. The granules are dried in the fluid bed granulator.
Operating Control Parameters Acceptable Ranges
Binder solution flow rate-1 1.0-1.6 kg/min
Atomizing air flow rate 1200-1600 L/min
Inlet air flow rate 15-60 m3/min
Inlet air temperature 80-100 °C
Outlet air temperature (Drying endpoint) (°C) 50-60°C
3 The dried granules are milled through a screening mill with a fixed screen size of 1.3mm.
Blending
4 Serratiopeptidase and a part of maize starch are mixed and milled through a screening mill with a fixed screen size of 1.3 mm.
5 The milled granules are blended with a screened serratiopeptidase and a part of maize starch in a diffusion mixer.
Operating Control Parameters Acceptable Ranges
Blending rotation count 33-77 counts
6 Remaining maize starch and magnesium stearate are mixed and screened through a sieve.
7 The first blended granules described in Step 5 are blended with the mixture described in Step 6 in a diffusion mixer.
In-process tests: Loss on drying, Particle size distribution, Bulk density, Yield Operating Control Parameters Acceptable Ranges
Blending rotation count 10-12 counts
Compression
8 The blended granules are compressed into tablets using a tablet press.
In-process tests: Appearance, Weight, Thickness, Hardness, Yield Operating Control Parameters Acceptable Ranges
Compression force 6.5-18.0 kN
Compression speed 20-45 min-1
Film Coating
9 Titanium dioxide is dispersed in ethanol, anhydrous by a homogenizer.
10 Poloxamers is dissolved in ethanol, anhydrous by a stirring.
11 Cellullose acetate phrthalate is dissolved in acetone and ethanol, anhydrous by stirring. Afterwards, the solution is mixed with the dispersion described in Step 9 and the solution described in Step 10 to prepare the film coating solution.
12
The uncoated tablets are film coated with the film coating solution.
Operating Control Parameters Acceptable Ranges
Inlet air flow rate 10-18 m3/min
Inlet air temperature 70-90 °C
Coating solution flow rate 250-280 mL/min/gun
Atomizing air flow rate 100-160 L/min/gun
Drum speed 4.0-8.0 min-1
The tablets are packaged into appropriate bulk containers. The tablets are then packaged into an appropriate commercial package defined.
4.0. REWORKING AND REPROCESSING
No reworking or reprocessing procedures are provided. If reprocessing should become necessary at some future date, the procedure will be submitted to the Agency for review and approval prior to use.
-8-